HRPP procedural change to reduce clerical burden
From: Alyssa Haase, HRPP Administrator; Susan MacNally, Director of Research Integrity
Sent: Thursday, Feb. 25, 2021, 1:30 p.m.
To: KU Lawrence human subjects PIs
Dear Human Subjects Researchers,
The Human Research Protection Program is changing requirements for continuing review for minimal-risk studies. These changes are consistent with federal regulations and aim to reduce clerical burden for investigators and streamline review processes while maintaining appropriate protections for human participants. This change is in accordance with 45CFR 46.109(f)(1).
Most minimal-risk studies approved after Feb. 28, 2021 will no longer require a periodic continuing review.
This change applies to studies that are currently approved under or transitioned to the 2018 Final Rule and have been determined to be of no more than minimal risk.
- Consent form stamps for studies released from the continuing review requirement will show an approval date only.
- The IRB retains authority to impose a continuing review requirement, depending on study details and with written justification. This is expected to be a rare occurrence for minimal-risk studies.
- Any modifications to the study must be submitted and approved prior to implementation, as usual.
Check your study in eCompliance to see if it is subject to 2018 or pre-2018 requirements.
Annual notification
In lieu of the continuing review, investigators of eligible minimal-risk studies approved under this change will receive an annual reminder of the obligations to submit modifications and reports of new information, as necessary, and to close any study that is not active.
Options for existing minimal risk studies that currently hold a 3- or 5-year approval period
- Modify: Any modifications to a study must be submitted and approved prior to implementation, as usual. If you need to modify your study, be sure to choose "Modification/Continuing Review" in eCompliance to both gain approval for the modification and release the requirement for continuing review.
- Convert: Submit a continuing review any time before your current expiration date to receive an updated consent form that does not require continuing review.
- Close: Submit a continuing review to close your study if it is no longer active.
No changes for more-than-minimal-risk studies
These new procedures apply only to minimal-risk studies approved after Feb. 28, 2021. The annual continuing review requirement still applies to more-than-minimal-risk studies, in accordance with 45 CFR 46.109(e).
A determination of more-than-minimal-risk is made by the full IRB at a convened meeting. The consent form and approval documents will have an expiration date, which indicates that continuing review is required.
Respectfully,
Alyssa and Susan
Alyssa Haase
HRPP Administrator
Susan MacNally
Director of Research Integrity
What requirements govern your study: 2018 or pre-2018?
On Jan. 21, 2019, revisions to human subjects regulations went into effect and are now known as the Final Rule, or 2018 requirements. Studies approved after January 2019 have been reviewed under the 2018 requirements. Studies approved prior to implementation of the 2018 requirements were reviewed under pre-2018 requirements and continue to be subject to the earlier requirements unless the principal investigator has submitted a modification to update the study to the 2018 requirements.
To determine if your study falls under the 2018 requirements or the pre-2018 requirements, you can check the regulatory authority listed on the main page of your study in eCompliance, as shown highlighted in yellow in the screenshot below.
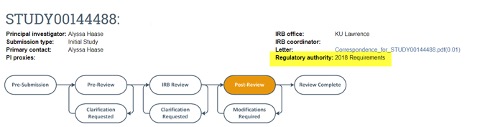
Investigators are encouraged to submit a modification in eCompliance to convert any study that remains under the pre-2018 requirements to the 2018 requirements.
Learn more about the 2018 revisions to human subjects regulations.