IRB submission process
If you plan to use human participants in your research, you are required to receive permission from the IRB before your project begins. Follow these steps to submit a study for review and continue your approved research over time. Scroll down to view the IRB meeting schedule and submission deadlines.
IRB submission details
The Collaborative Institutional Training Initiative, or CITI, provides all KU researchers and associates with access to an expertly updated human subjects research tutorial. The tutorial must be completed every three years with a cumulative score of 80% each time.
New study checklist
Access human subjects research forms.
- Consent forms
- Assent forms (minors)
- Recruitment materials
- Interview/survey/focus group questions
- Tests/assessments
- Debriefing statement (deception or omission studies)
- External site approval letter
- KU Environmental Health & Safety approval
- HIPAA documents
- Award/contract materials
- Other relevant forms
- Required once every 3 years.
- Faculty supervisors need to have current human research training for student projects (all study team members need training).
- Contact irb@ku.edu to receive assistance with external study team members.
- Single-site: Only KU-Lawrence researchers (personnel or investigators under an individual investigator agreement) are conducting research procedures. Example: KU-Lawrence personnel only are conducting interviews at one or more research locations.
- Multi-site: KU-Lawrence researchers and researchers from another institution both will be conducting research procedures. Example: KU-Lawrence personnel are conducting interviews. University of Missouri collaborators also will be conducting interviews.
- Undergraduate students may not have access to eCompliance. Contact irb@ku.edu for assistance.
- Upload all relevant documents.
- Add study team members (including faculty supervisor).
- Submit using the "Submit" button on study homepage.
- Students only: Have faculty supervisor complete ancillary review.
Once you've submitted your study, the Human Research Protection Program will determine whether it meets the criteria for human subjects research, whether it presents minimal or greater risk for participants, and what category of review is required.
Create a modification request in eCompliance
- Log in to KU's eCompliance website using your KU ID and password.
- Access your study by clicking the "IRB" link the red banner, then clicking the "All Submissions" tab.
- Click the "Create Modifications/CR" button.
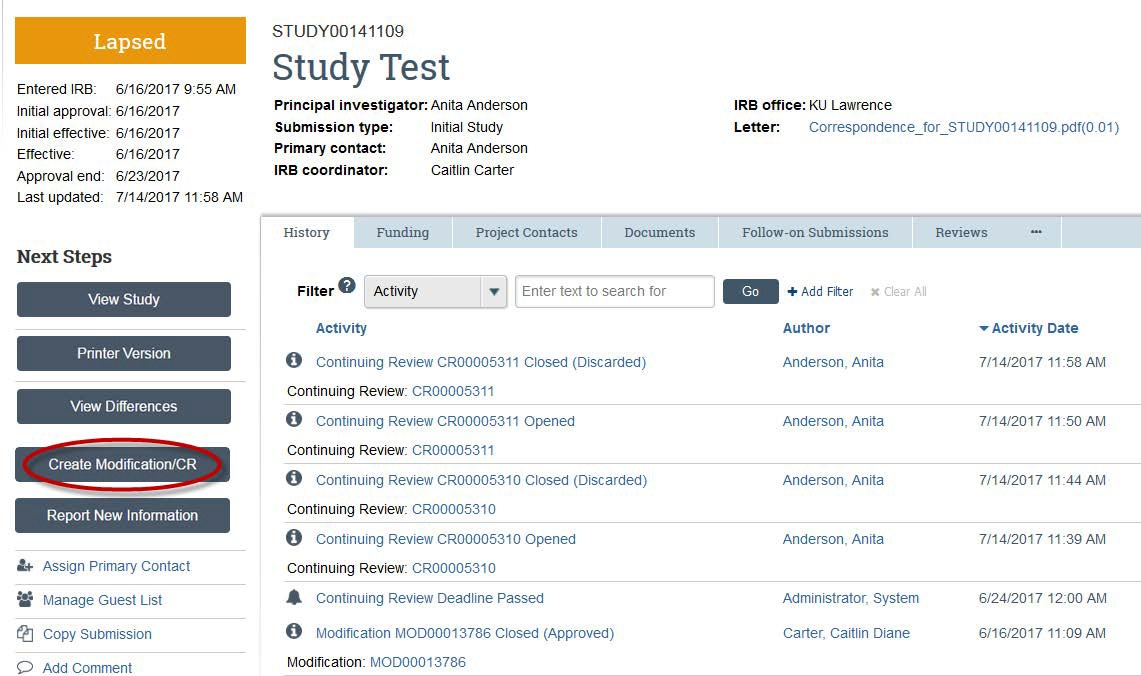
- Choose "Modification" if you want to change some part(s) of your study.
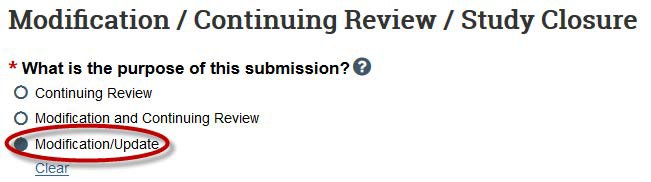
- Next, select the scope of the change.
- Select "Study team member information" if you wish to add/delete study team members.
- Select "Other parts of the study" to edit study documents, change study procedures, add external sites, or add a funding source.
- If you are trying to change the PI, please consult our Change the PI guide.
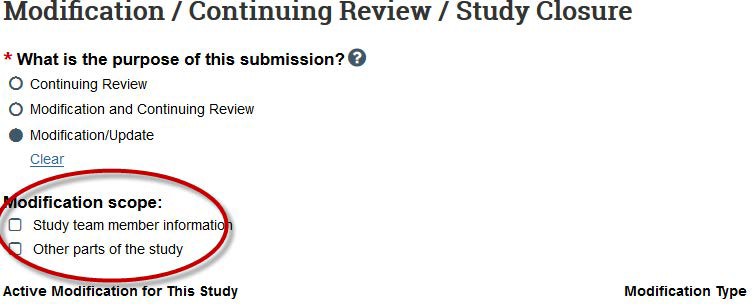
- On the Modification Information page, provide information about the changes you are requesting. Check all boxes that are relevant to your modification.
Use the “Summarize the modifications” section (required) to clearly describe the changes you are requesting.- In lay terms, provide a point-by-point explanation of all of the changes to your study.
- Summarize the reasons for the changes.
- Explain if the changes will increase/decrease risks to research participants.
- Explain how/if participants will be notified of the changes to your study procedures.
- List the documents included in the submission.
- If you clicked the “Other parts of the study” and/or “Study team member information” scope, you can now edit the original local site documents and upload new documents, edit study team members, add funding sources, etc. If your study is a multi-site study, you also will have the option to edit and add study-related documents.
To reduce IRB review time, upload both a tracked changes version and a clean version of any edited documents.
- Click “Finish” on the last page or “Save” and “Exit” from the menu.

Create a continuing review in eCompliance
- Log into KU's eCompliance website using your KU ID and password.
- Access your study by clicking the "IRB" link the red banner, then clicking the "All Submissions" tab.
- Click the "Create Modifications/CR" button.

- Choose "Continuing Review."
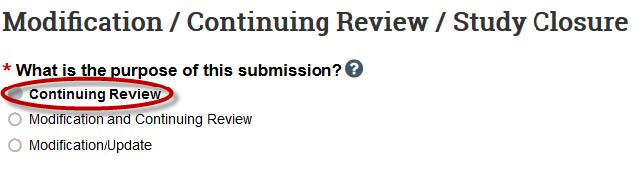
- Complete the Continuing Review Information page.
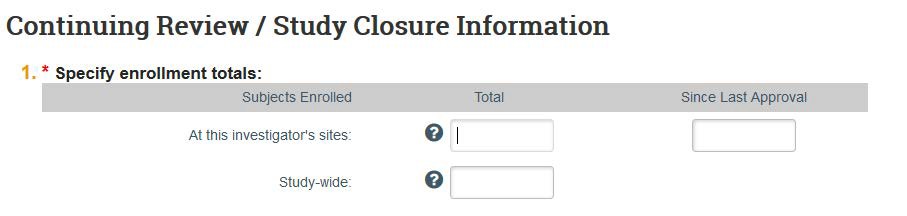
5a: Indicate how many people have participated in your study.
- At this investigator’s sites: For multi-site studies, add only the number participants for KU. If only KU is participating in this project, this number will be the same as your “Study-wide” total.
- Study wide: For multi-site studies, add the total number of participants across all sites. If research is only taking place at KU, this number will be the same as the “Investigator sites” total.
- Since last approval: Amount of people who have participated since your last continuing review (CR) was submitted. If this is your first CR, this number will be the same as your “Study wide” total.
5b: Check only the research milestones that apply to this specific study. If none apply, you are not required to check any boxes.
5c: Indicate whether study team members, including the PI, have any financial interests related to this study.
5d: The next section helps the IRB determine if the risks and/or benefits have changed since you originally submitted the study. In most cases, all the boxes should be checked to confirm that the statements are true.
5e: If any boxes were left unchecked, submit a document that explains why. Do not upload consent forms or any other study-related materials here.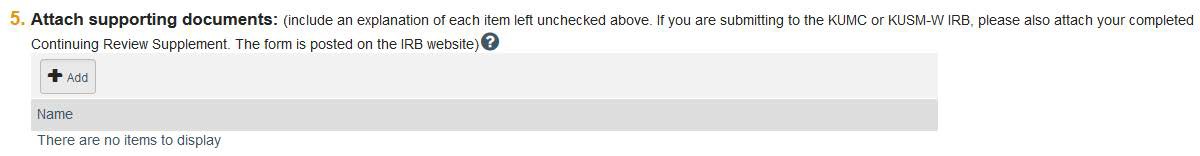
- Click the "Continue" button.

- Click the "Finish" button.

- In order to submit your continuing review for evaluation by HRPP staff, click the "Submit" button on the left side of the screen. If you are not the PI on the project, then the PI will need to log in and click the "Submit" button.
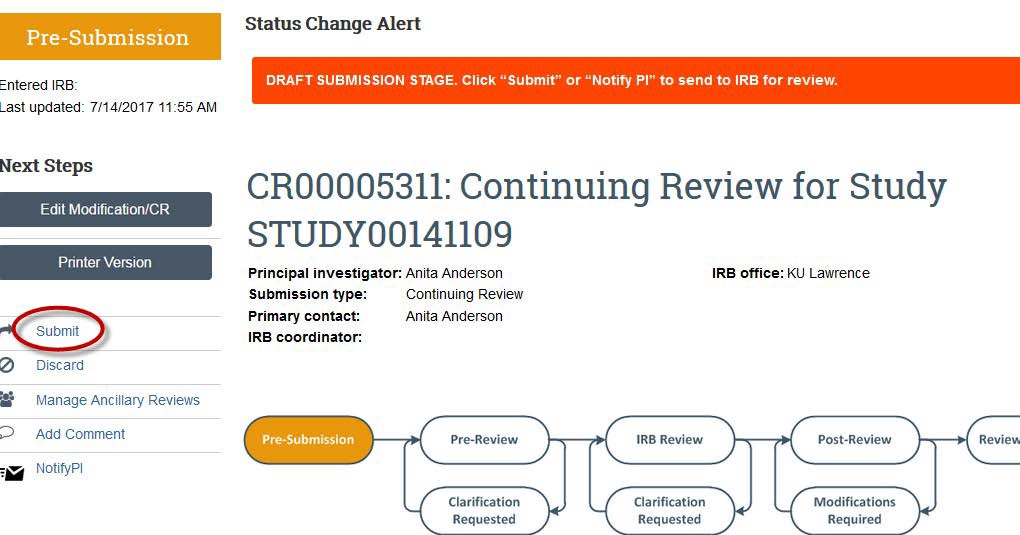
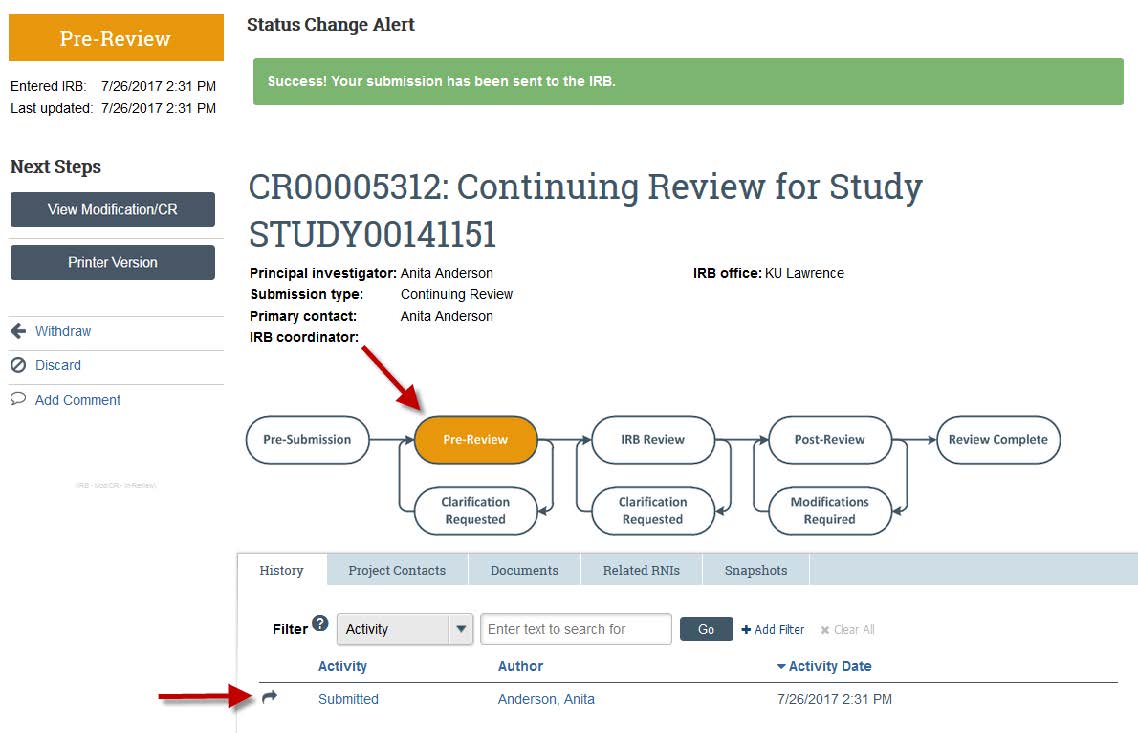
- Once the study is submitted, the flowchart will change from “Pre-Submission” to “Pre-Review” and the History Activity will show that the study has been submitted. There also will be a green banner at the top of your screen for a few seconds to show submission.
Close a study in eCompliance
- Log into KU's eCompliance website using your KU ID and password.
- Access your study by clicking the "IRB" link the red banner, then clicking the "All Submissions" tab.
- Access your study by clicking the hyperlinked study title. Click the "Create Modifications/CR" button.

- To close a study, choose "Continuing Review."

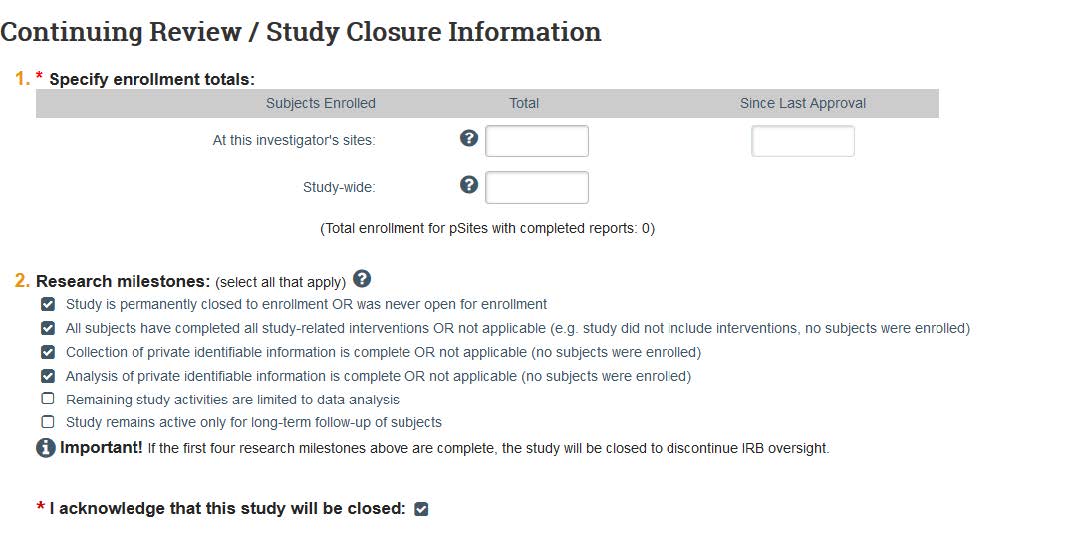
- On the next page, enter the number of participants in Question 1, and then click at least the first 4 milestones on Question 2. If these 4 milestones are not true for your study, then you should not close your project:
- Study is permanently closed to enrollment OR was never open for enrollment.
- All subjects have completed all study-related interventions OR not applicable (e.g. study did not include interventions, no subjects were enrolled).
- Collection of private identifiable information is complete OR not applicable (no subjects were enrolled).
- Analysis of private identifiable information is complete OR not applicable (no subjects were enrolled).
- Complete the rest of the information in the form and click “Finish.” In order to submit your study for review by HRPP staff, click the “Submit” button on the left side of the screen. If you are not the PI on the project, then the PI will need to log in and click the “Submit” button.
The system will require that you enter your KU ID and password.
- Once the study is submitted, the flowchart will change from “Pre-Submission” to “Pre-Review” and the History Activity will show that the study has been “Submitted." There also will be a green banner at the top of your screen for a few seconds to show submission.

KU Medical Center submissions
You will need to submit your study to the KUMC Human Research Protection Program rather than the Lawrence program in the following instances:
- If your study will be conducted solely or in part on the KUMC campus.
- If your research uses KUMC non-public information to identify, contact or recruit human subjects or prospective subjects.